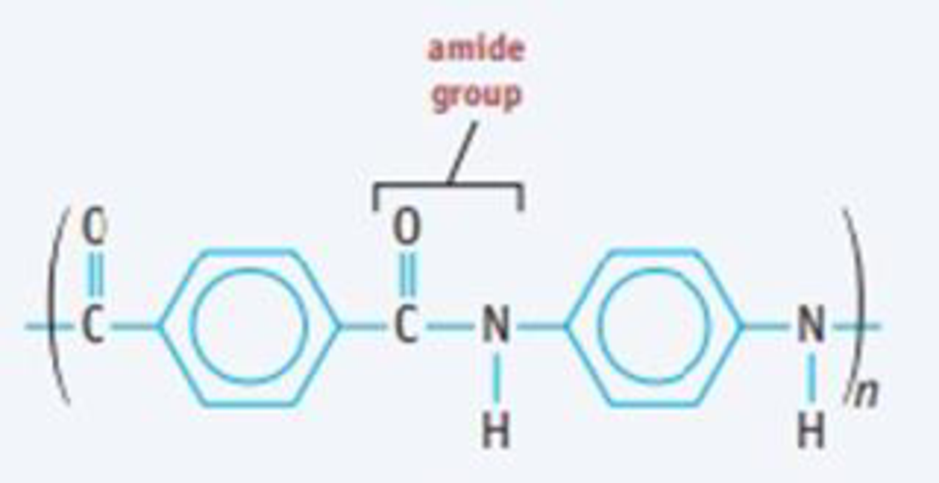
Kevlar, a polyamide used to make bulletproof vests, is made from terephthalic acid and paraphenylenediamine. Write a condensed (bracketed) repeating structure of the Kevlar molecule.
$ 26.50 · 4.9 (127) · In stock

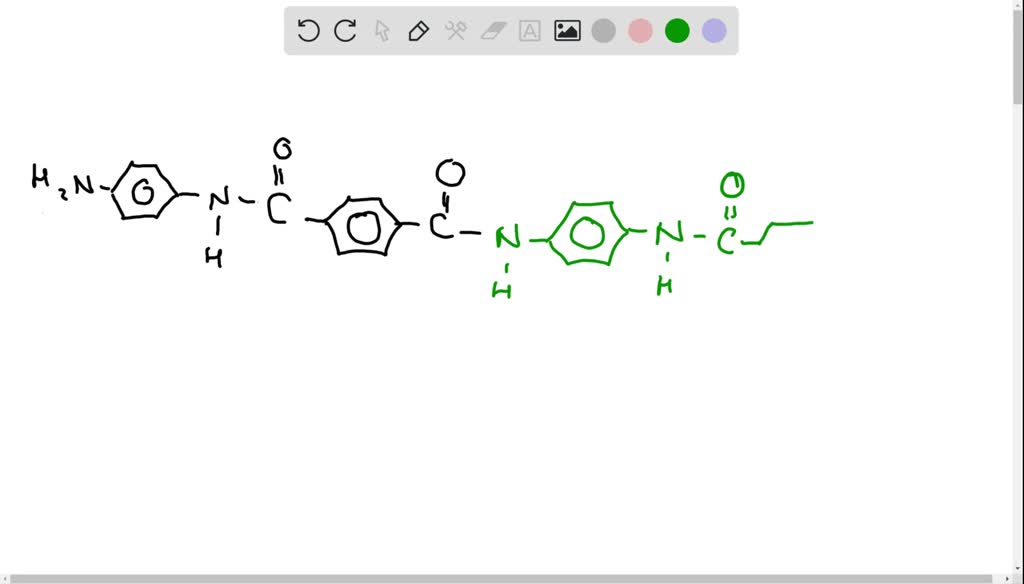
SOLVED: The Kevlar that is used in bulletproof vests is a condensation polymer that can be made from the following monomer. Draw a portion of a Kevlar polymer showing four molecules of

Suppose the given glycol is used with terephthalic acid to form a polyester. Write the structure of the polymer, showing two repeating units.
If you had enough layers of Carbon Fiber Kevlar mixed fabric, would it be bullet proof? - Quora

Plastic photochromic sunglasses are based on the following reversible rearrangement of a dye inside the lenses that occurs when the lenses are exposed to sunlight. The original dye absorbs UV light but
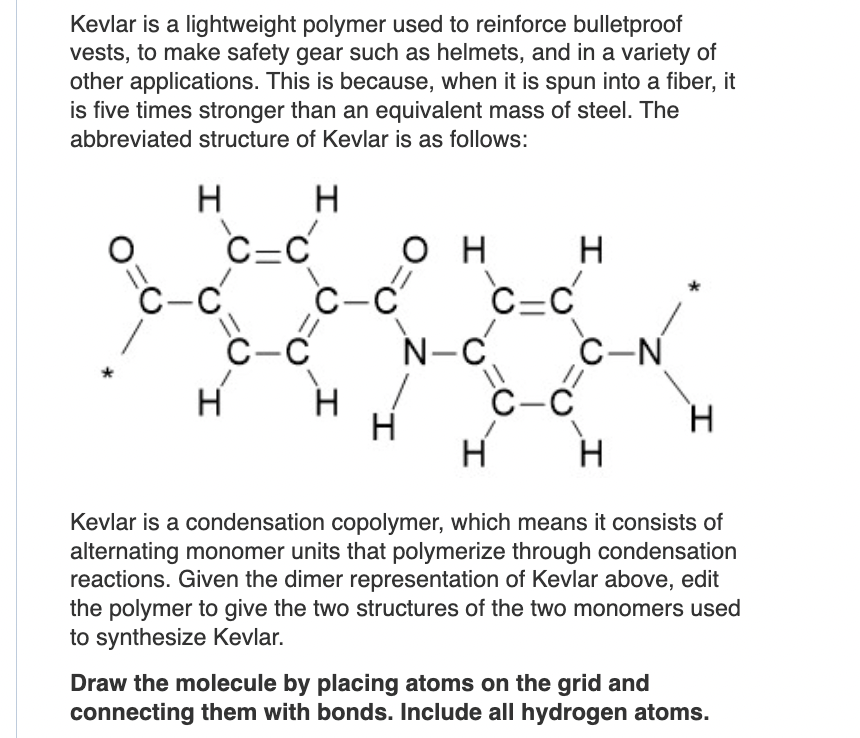
Solved Kevlar is a lightweight polymer used to reinforce

Kevlar is a polymer fiber used to make bulletproof vests. What are the structures of the monomer units in this polymer?

Write a condensed structural formula for the repeat unit of the Kevlar molecule
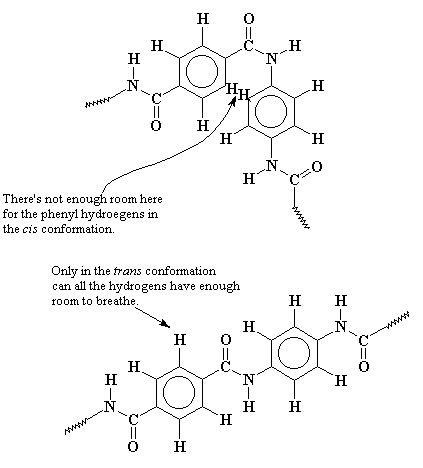
Kevlar - Molecule of the Month November 2010 - JSMol version

Neoprene is an additional polymer of chloroprene: Write the structural formula of neoprene.

Kevlar --Twaron--poly-paraphenylene terephthalamide

Kevlar - Wikipedia